Quality Safety
Basic approach and policy
In order to meet customer requirements for products that are safe and reliable to use, the Group has established a quality assurance system and prioritizes the supply of safe and reliable products.
Quality & Food Safety Policy
We will implement the following policies to provide customers with reliable products.
- We value communication with our customers and we work to provide satisfying products that embody their needs.
- In all processes, from R&D to raw material procurement, manufacturing, distribution, sales and administration, we steadily fulfill our roles and duties and provide quality products and services that our customers rely on.
- We observe related laws and regulations, establish a quality assurance system that allows us to provide safe, high-quality products, and work continuously to make improvements.
Relation with stakeholders
We are committed to providing products that customers can use with a sense of security and that are valuable for customer success.
Governance and risk management
To ensure customers and consumers of the quality and safety of our products, we have built a quality assurance system led by the Quality Assurance Division, which communicates directly with the President & COO. We value communication with customers, and we promote quality and safety activities. All raw materials that we use are registered under the system. Only raw materials whose quality and safety have been verified are used to make sample pieces and products. We focus on providing high-quality products while also performing final inspections for all customers’ products.
Quality assurance system chart
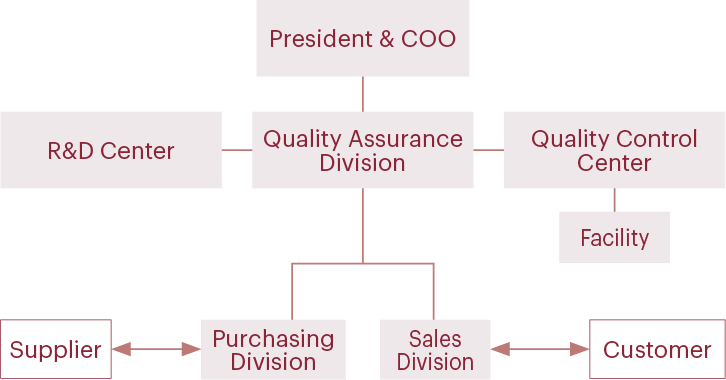
Our quality assurance system
Quality Assurance Division
We confirm legal compliance at the raw material purchase stage to provide customers with products that they can use with peace of mind. In addition to complying with laws and regulations in Japan, we check laws and regulations of destination countries of export products for our overseas customers and provide products that comply with the laws and regulations of the respective countries and regions.
We also promote automatic creation (digitalization) of product specifications so that we can quickly provide customers with accurate information.
Quality Control Center
The Quality Control Centers of the Fukaya Facility and the Itakura Facility manage the plant quality system and conduct quality inspections of products manufactured at the facilities and purchased raw materials.
In quality inspections, we have established a system that allows us to carry out all inspections internally. Inspection results are automatically transferred from the inspection equipment to a computer system by an automatic transfer system. Measurement, pass/fail determination, and inspection card issuance are fully automated (digitalized), which helps to prevent transcription errors. As a company that handles scents, we are also working on training sensory inspectors. Only those who have been trained to distinguish scents and have passed our internal certification test are involved in sensory tests.
Communication with customers
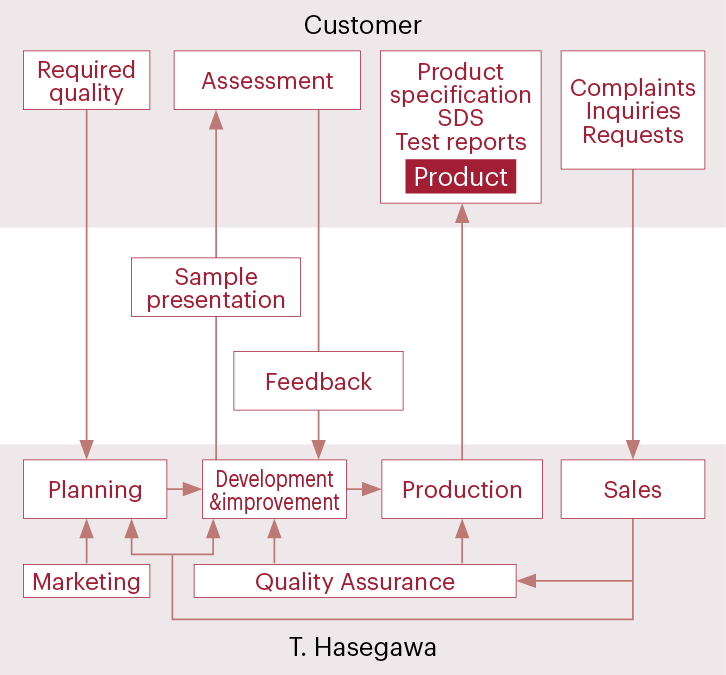
Quality control from raw materials to products
We use a traceability system based on barcodes for processes from raw material acceptance to product shipment to ensure product quality so that our customers can use our products with a sense of security. All information is traceable under the system, from product lots to manufacturing records, product inspection results, lot numbers of raw materials used in the products, and raw material acceptance inspection results.
We check the quality and safety information for the raw materials that we use, including standards, allergies, and residual agrochemicals. We also confirm the compliance of compounds used in flavors and fragrances with related laws, including the Food Sanitation Act, the Fire Service Act, the Industrial Safety and Health Act, and the Act on the Regulation of Manufacture and Evaluation of Chemical Substances. By consistently using raw materials with guaranteed quality and creating quality products, we provide safe and reliable products and manufacture products while considering the environmental impact.
In addition, a traceability system allows information to be provided promptly in response to customer inquiries.
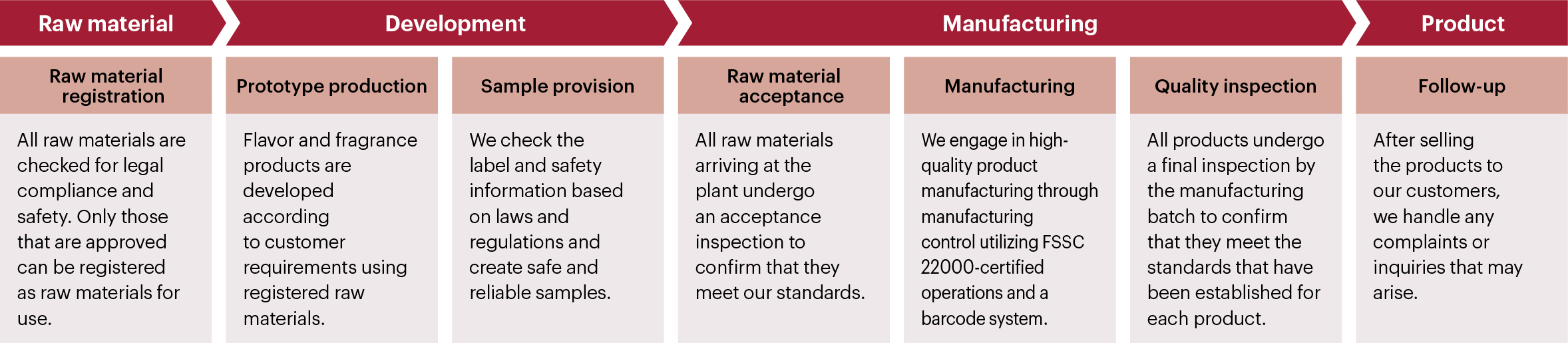
Laws related to chemical substances and our compliance
From the viewpoint that the components of flavors and fragrances we handle are chemical substances, we actively and appropriately comply with related laws to protect the workers who are handling chemical substances during manufacturing and transport, ensure the safety of users, and conserve the environment.
1.Application for registering new chemical compounds in accordance with Japanese laws
We perform the following in compliance with the Act on the Regulation of Manufacture and Evaluation of Chemical Substances:
- Notification concerning “Low Production Volume of New Chemical Substances”
- Request pertaining to “Small Production Volume of New Chemical Substances”
We perform the following in compliance with the Industrial Safety and Health Act:
- Application and registration of “New Chemical Substances”
- Application for “Small Production Volume of New Chemical Substances”
2.Publication of Safety Data Sheets (SDSs) supporting the Globally Harmonized System of Classification and Labelling of Chemicals (GHS)
We issue SDSs and disclose information on substances subject to the law contained in flavor and fragrance products for all of our products so that customers will be able to use them safely. Applicable related laws are as follows:
- Industrial Safety and Health Act
- Act on the Assessment of Releases of Specified Chemical Substances in the Environment and the Promotion of Management Improvement (Chemical Substances Management Law)
- Fire Service Act
- Ship Safety Act
- Act on Prevention of Marine Pollution and Maritime Disaster (Marine Pollution Prevention Law)
- Poisonous and Deleterious Substances Control Law
For products to be exported, we also issue SDSs corresponding to the GHS system of the export destination country.
3.Information management and response to chemical regulation trends in countries and law amendment in Japan
We obtain information on chemical-related regulation trends of countries and items listed in the registered chemical substance list (inventory) through law search databases in and outside Japan, as well as through industry organizations. We manage the chemical substances by registering information on how they are obtained in our internal system, and we use this data to respond to inquiries from customers.
As for the amendment of laws in Japan, we work to obtain notifications from relevant government offices as well as industry organizations. We collaborate with relevant divisions by responding to amendments to the Industrial Safety and Health Act and the Chemical Substances Management Law, which have frequently been amended, especially in recent years, when collecting raw material information and improving internal systems. We do so while considering the fact that the frequency of counting the emissions of flavors and fragrances applicable to the Pollutant Release and Transfer Register (PRTR), updating SDSs, and changing label indication, as well as the number of materials subject to risk assessment, will further increase in the future.
4.Response of overseas subsidiaries
Our overseas subsidiaries are also responsible for complying with chemical-related laws applicable to each site.
China
-
①Main chemical management regulations applicable to our subsidiaries in China
(Shanghai Facility and Suzhou Facility)
Safety production regulations: Work Safety Law of the People’s Republic of China; Fire Control Law of the People’s Republic of China; Regulations on the Control over Safety of Hazardous Chemicals; Measures for Administration of Hazardous Chemicals Registration; Catalog of Hazardous Chemicals; Safety Production License Ordinance; National Centralized Management Plan for the Safety Risk of Hazardous Chemicals; Notice on the Informatized Management of the Entry and Exit of Hazardous Chemicals and the Loading and Unloading of Hazardous Materials Based on the One Enterprise One Product One Code Policy (Shanghai Emergency Hazardous Chemicals [2024] No. 12)
Occupational health and safety: Law of the People’s Republic of China on Prevention and Control of Occupational Diseases; Regulations on the Management of Occupational Health at Workplaces; and Warning Signs for Occupational Hazards in the WorkplaceEnvironmental protection regulations: Environmental Protection Law of the People’s Republic of China; National Inventory of Hazardous Wastes; and Measures on the Management of Transfer of Hazardous Waste -
②Application for registration and notification of new chemical substances
T. Hasegawa Flavours and Fragrances (Shanghai) Co., Ltd. has so far applied for a simplified single item registration in accordance with the “Measures for Environmental Management of New Chemical Substances” (Order No. 7 of the Ministry of Environmental Protection) and for notification of a second item in accordance with the “Measures for the Environmental Management Registration of New Chemical Substances” (Order No. 12 of the Ministry of Ecology and Environment; enforced in 2021). -
③Submission of SDSs
We issue SDSs for all products that comply with GB 30000: Rules for Classification and Labeling of Chemicals, GB 12268: List of Dangerous Goods, and GB 2944: Classification and Code of Dangerous Goods.
Malaysia
-
①Main chemical management regulations in our subsidiaries in Malaysia
Industrial Safety and Health Act 1994 (OSHA 1994)
Subordinate legislation of OSHA 1994: Occupational Safety and Health (Use and Standard of Exposure Chemical Hazardous to Health) Regulations 2000 (USECHH Regulations)
The Malaysia Facility only manufactures food flavors, so it does not have to register chemical substances. However, we internally manage records of substance names listed in the inventory of hazardous harmful chemical substances of CLASS Regulations 2013 (2013 Regulations on Classification, Labelling and Safety Data Sheets of Hazardous Chemicals). -
②Submission of SDSs
We issue SDSs that comply with the CLASS Regulations 2013. We obtain SDSs of chemical substances used as raw materials of food flavors from suppliers and issue SDSs for samples and products according to customer requests.
United States
-
①Main chemical management regulations applicable to our subsidiaries in the United States (2 sites
in California and 1 site in Illinois)
Our subsidiaries in the United States only manufacture food flavors and not new chemical substances, so they are exempted from the law on applying for registration of new chemical substances. Under the chemical substance management law, Proposition 65 and the Emergency Planning and Community Right-to-Know Act (EPCRA) apply, specifically the Toxics Release Inventory (TRI) Tier II reporting requirement at our Illinois facility only. For Proposition 65, we provide statements prior to the customer’s initial order for the product or at the customer’s request. -
②Submission of SDSs
We issue SDSs in compliance with the GHS for samples and products according to customer requests.
Product risk assessment (risk assessment of chemical substances)
Factory employees regularly receive education on the danger, harm, and appropriate handling of chemical substances and chemical regulations in and outside Japan in order to appropriately manage the chemical substances handled by the Company as raw materials. In addition, we conduct risk assessments of chemical substances based on the SDSs of raw materials and intermediate products, consider risk reduction measures, and reflect the results in work procedures. We warn all workers who handle chemical substances of the toxicity of chemicals, provide precautions for handling chemicals, and provide exposure prevention measures (by installing local exhaust ventilation and wearing protective equipment).
The R&D Center conducts risk assessments of all chemical substances used in the factories according to the Industrial Safety and Health Act, prepares a measure sheet according to the risk level, and puts it on the intracompany website so that it will be available to employees at all times. We will also respond to an increase in substances subject to risk assessments in the future due to amendments to the Industrial Safety and Health Act.
At our overseas subsidiaries, we also take action according to the actual situation there.
China
The Shanghai Facility and the Suzhou Facility independently manage chemicals according to the respective local requirements. They formulate and manage the following management measures from the perspectives of safety production, occupational health and safety, environmental protection, and production risk management.
- ①Obtain SDSs from raw material suppliers and register the information so that it can be searched on internal systems.
- ②Evaluate workshops in accordance with legal requirements to identify occupational hazards. Regularly inspect workshops every year, publicly announce the results, and put occupational health warning marks at workshops.
- ③Store flammable, dangerous chemicals in specified warehouses.
- ④Conduct a safety production risk assessment once a year to identify risks (such as the use, storage, and transport of dangerous chemicals) and establish control measures and emergency countermeasures to prevent production accidents from occurring.
- ⑤Register information on fragrances produced at the Shanghai Facility and imported hazardous chemical substances on the website of the National Registration Center for Chemicals, generate QR codes, obtain updated registration certificates, and manage them in accordance with relevant requirements.
Malaysia
In order to confirm that a risk assessment has been conducted for all chemical substances and to ascertain the impact of such substances on employees, our subsidiaries in Malaysia receive chemical substance health risk assessments by external authorized reviewers registered with the Department of Occupational Safety and Health (DOSH) of Malaysia in accordance with USECHH Regulations. Based on the risk assessment results and in accordance with relevant regulations, we installed local exhaust ventilation devices (fume hoods) at research labs and employed management measures to confirm the permissible exposure limit of employees. In addition, we provide protective equipment to employees approved by the Standard and Industrial Research Institute of Malaysia-Department of Occupational Safety and Health (SIRIM-DOSH).
United States
At our subsidiaries in the United States, risk assessments of raw materials are conducted based on the hazard risk information for respiratory organs of the Flavor and Extract Manufacturers Association of the United States (FEMA) or other information provided by suppliers. All employees receive hazard communication training. The facilities comply with the requirements of government agencies and the Division of Occupational Safety and Health (OSHA). Regarding exposure to employees’ respiratory organs during flavor manufacturing, we observe the priority chemical substance list for health and safety provided by FEMA. Precautions on safety are all reflected in manufacturing process sheets so that employees can observe them.
International standard certification
ISO9001
In 1998, we acquired an ISO 9002 certification for the quality management system at our facilities. In 2003, we changed the registration to an ISO 9001 certification. In 2007, we expanded the scope and acquired a certification covering the entire Company. With the strong leadership exemplified by the President & COO as top management, all employees involved in business activities cooperate in quality activities.
In 2012, we obtained a FSSC 22000 certification for our food safety management system and then began rolling out an integrated management system of ISO 9001 and FSSC 22000. However, in order to narrow down the activities at each division and focus on the most suitable standards, we have changed the scope of ISO 9001 to cover the head office, branches, sales offices, R&D Center, and fragrance facilities with the certification and to cover flavor facilities with only the FSSC 22000 certification since 2019.
In China, we acquired an ISO 9001 certification in Shanghai and Suzhou in 2004 and 2010, respectively. These certifications continuously guide our quality activities.
FSSC22000
In 2012, all divisions associated with flavors obtained an FSSC 22000 certification for their food safety management systems. We acquired an international standard certification approved by the Global Food Safety Initiative (GFSI), which allowed us to reduce risks related to food safety and provide safe and reliable products that are recognized by our customers overseas and in Japan.
Since the FSSC 22000 standard specializes in food safety, we have changed the scope of the certification and have been obtaining it only at our flavor facilities since 2019.
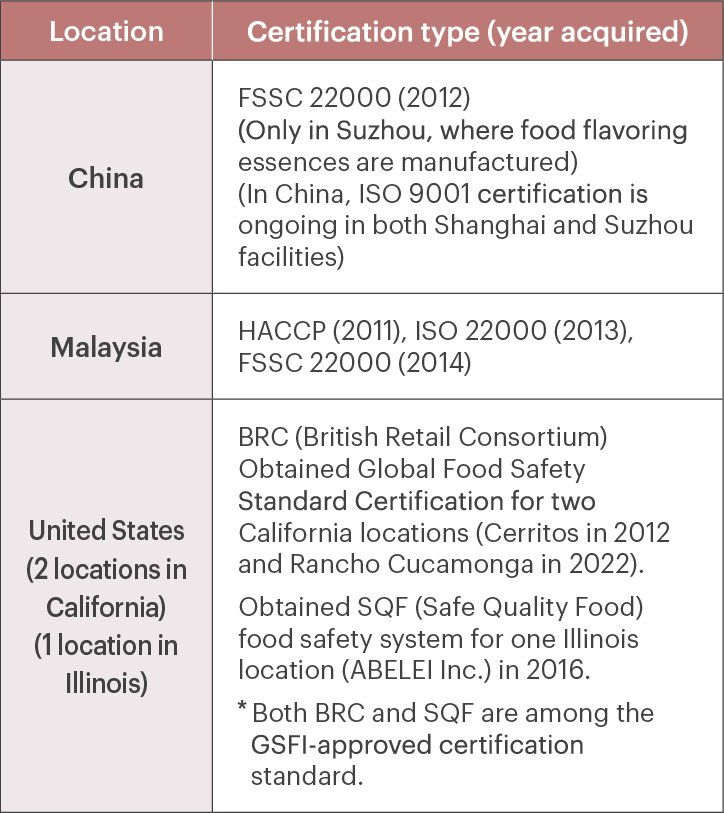
We have also acquired certifications under international standards related to food safety at our overseas subsidiaries.
Overseas subsidiaries - Status of international food safety standards certification
Audit
Internal audit
We conduct the annual internal quality and food safety audit at all divisions subject to international standard certification. We provide specialized training to some employees, as well as those approved as internal auditors. By having employees audit one another, we can make detailed improvements to areas that we do not usually notice and enhance the efficiency of our operations. Findings are shared with other divisions to make ongoing improvements in every division.
5S Audit
Members from the Quality Control Center of the Fukaya Facility and the Itakura Facility enter the manufacturing area to conduct hygiene audits based on the 5S, which stands for Seiri (Sort), Seiton (Systemize), Seiso (Shine), Seiketsu (Standardize) and Shitsuke (Sustain), by using a method equivalent to that used in external audits according to ISO 9001 or FSSC 22000. The hygiene control of a factory forms the basis of food safety and helps prevent contamination by foreign matter or microorganisms.
In China (Shanghai and Suzhou), regular patrols and audits are conducted by the 5S Management Committee.
Management review
To provide information to management, we provide reports to review activities related to quality and food safety performed during the year. We conduct ongoing improvement activities by reflecting on the year’s activities and receiving approval and assignments from management.
Religious Compliance Committee
To accommodate customers in Islamic regions with halal-certified products, as well as kosher-certified products in accordance with Jewish dietary laws, we have established a Religious Compliance Committee. The committee consists of members selected from relevant departments, including sales, R&D, production, and quality assurance. We also work closely with our overseas affiliates and external organizations to ensure proper management and ongoing compliance with halal and kosher certification requirements.
Response to complaints and the Quality Control Committee
When a complaint arises, quality-related divisions coordinate with each other to clarify the cause and countermeasures and quickly respond to the customer. Serious complaints are promptly reported to management, and proper measures are taken from the customer’s perspective. Complaint information is shared at Quality Control Committee meetings and with related divisions to help prevent recurrence. When a manufacturing problem occurs, the cause and countermeasures are clarified in the same way as when dealing with complaints, and information is shared at Quality Control Committee meetings to help prevent recurrence.
Strategies
Increase in business opportunities
- Increased trust owing to improved product quality
Measures
- Investing in aging equipment
- Improving the skill level of inspectors
- Reviewing methods and manufacturing conditions
- Improving the manufacturing skill level through education and training
- Enhancing the production system (supply system)
- Reducing the lead time by promoting fully automated issuance of inspection cards (digital transformation)
- Developing the sensory skill of manufacturing personnel to differentiate taste and scent through sensory test division training
Customers will always require safety and security. We will work to enhance our quality assurance system and aim to achieve a level of quality that customers will continue to rely on. The Quality Assurance Division, which communicates directly with the President & COO, will lead our efforts to ensure close collaboration with the Quality Control Center of the Production Division and the Quality Assurance Divisions of our overseas locations to provide quality products that satisfy all customers both in Japan and abroad. At our factories, we will review the manufacturing methods and work to improve operations in order to reduce the number of complaints and further reduce the rejection of goods. In addition, we will conduct factory-wide activities to review work procedures and promote the visualization of work and stabilization of quality. We will continue to obtain the ISO 9001 and FSSC 22000 certifications to improve quality and maintain food safety.
Furthermore, the Production Division will proactively provide training for skill improvement at the R&D Center to develop highly skilled workers who can take on the responsibility for manufacturing high quality products. At the Quality Control Center, we will work to improve the skills of inspectors through education and training and enhance the inspection system with the aim of providing customers with high-quality products.
In our overseas locations, we will also continue to train employees involved in production, introduce an operational improvement suggestion program, and engage in process control in the Production Divisions in the same manner as the Company.
We also realize that properly managing chemical substances and considering the health and environment of our employees and local residents are important issues in building a sustainable society.
Going forward, we will continue to monitor trends in regulating related chemicals, including amendments to the Industrial Safety and Health Act, conduct risk assessments of applicable chemical substances, and reflect changes to SDSs.
Continued enhancement of the promotion system
Strengthening the development of the flavor and fragrance industry and responding to the environment surrounding the industry
We actively dispatch employees to serve as members of industry organizations in other countries in an attempt to respond to constantly changing legal information and develop the flavor and fragrance industry as a whole. We also joined organizations in Japan, including the Japan Flavor & Fragrance Materials Association (JFFMA) and the Japan Food Additives Association, as well as international industry organizations, such as the International Fragrance Association (IFRA) and the International Organization of the Flavor Industry (IOFI), as a special member of the JFFMA. We take quick and appropriate action for the entire industry regarding various regulations related to the industry and conduct activities that will help develop the entire flavor and fragrance industry.
In addition, we have joined the China Association of Fragrance Flavor and Cosmetic Industries (CAFFCI) in China and FEMA in the United States, and we dispatch employees as committee members to participate in the activities of industry organizations.
Enhancement of response to quality requirements in R&D
Various legal regulations are constantly changing, not only in Japan but also overseas. In order to promptly respond to such changes, reflect them in products, and communicate the information to customers, it is necessary to participate actively in activities of industry organizations to collect information. The collected information is registered in an internal database and quickly reflected in product information. Updated information can always be referenced by researchers, which allows them to develop products based on the latest information, and is also used to provide information to customers.
Enhancement of response to quality requirements in manufacturing processes
In the manufacturing process, we are always required to produce products with consistent quality. We assign codes to every raw material and product. By managing them using barcodes, we prevent incorrect raw materials from being input and products from getting mixed up. In our compound flavor and fragrance facilities and powder facilities, the system records measurement data from scales used to measure raw materials and fill products. The data is used in combination with barcode management to help manage manufacturing and ensure traceability. By installing IT in our facilities, we consistently provide products with the specified quality.
In FY2024, regular audits for ISO 9001 and FSSC 22000 were conducted continuously, and we received approval for maintaining the certifications with no serious findings. Audits have also been conducted and renewals granted for Halal and Kosher certifications. As an initiative for food safety, we pruned trees within the factories’ premises and strengthened rat-proof measures by installing rat-proof devices. The raw materials that we use are delivered from around the world. As such, we strengthened the acceptance and inspection work in cooperation with the Procurement Division. We worked to improve our services by promoting the direct delivery of samples from factories to customers with whom we have close business relationships, thereby increasing our response speed.
Employees engaged in the manufacturing of products for foods take courses on HACCP and work to gain knowledge on and raise awareness of food safety.
We also work to increase awareness of quality and food safety system requirements and improve the operations of standards by the employees of each division by providing courses on ISO 9001 and FSSC 22000.
Compliance initiatives
We believe employee awareness is important to provide customers with high-quality products, and we are engaged in activities to raise compliance awareness. We repeatedly provide quality management training on various occasions, including when employees join the Company and when they are promoted. In particular, legal information changes on a daily basis, so we constantly collect information from industry organizations and various media and work to promptly communicate legal information to employees through intracompany websites, regular workshops, and various study sessions. Such information is posted on intracompany websites so employees can check these details at any time. Regular study sessions on food safety are held at the Fukaya Facility and the Itakura Facility to raise compliance awareness among employees.
Indicators and targets
The Group ensures strict control of manufacturing processes and provides training for employees in order to
maintain its ISO 9001 and FSSC 22000 certifications. In FY2024, there were zero serious quality incidents
requiring market recalls.
For more details, please refer to
the T. Hasegawa Group ESG Data Book (PDF)
.
